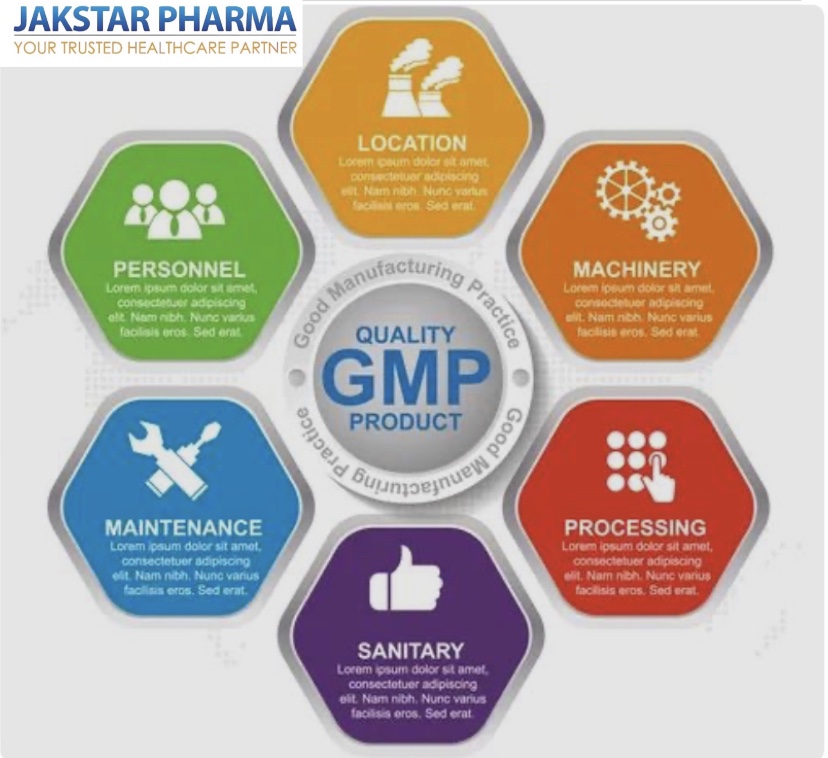
Good Manufacturing Practices (GMP) :
They are essential to ensuring the quality and safety of medicines. These practices are designed to minimize the risks involved in pharmaceutical production that cannot be eliminated through testing the final product.
GMP guidelines provide a set of minimum requirements for the manufacture of pharmaceuticals, including the selection of raw materials, the use of equipment, the cleanliness of the manufacturing environment, and the training of personnel. The aim of GMP is to ensure that pharmaceutical products are consistently produced and controlled to meet the quality standards appropriate to their intended use.
Manufacturers must implement GMP procedures in their production processes to prevent contamination, errors, and deviations from established quality standards. This ensures that the final product is safe, effective, and of high quality.
In summary, GMP guidelines are essential for ensuring the safety and efficacy of pharmaceutical products. It is important for manufacturers to adhere to these guidelines to ensure that the medicines produced are of the highest quality, and to minimize the risks to patients.